Research
Aktivation of Small Molecules: Dinitrogen and Carbon Dioxide
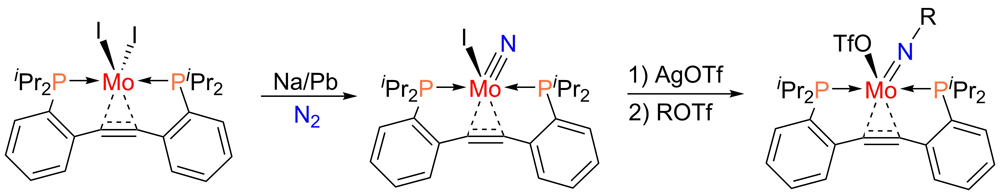
For the activation and functionalization of N2, we have set our focus on diphosphinotolane complexes, which were shown to exhibit a certain degree of non-innocence with regard to alkyne-metal interaction. Recently, we have demonstrated that gaseous dinitrogen may be cleaved using a diphosphinotolane-coordinated molybdenum complex. The coordinated alkyne within the ligand backbone is not reduced over the course of this process. The alkyne unit is also tolerated in follow-up functionalization reactions as shwon in the figure below.
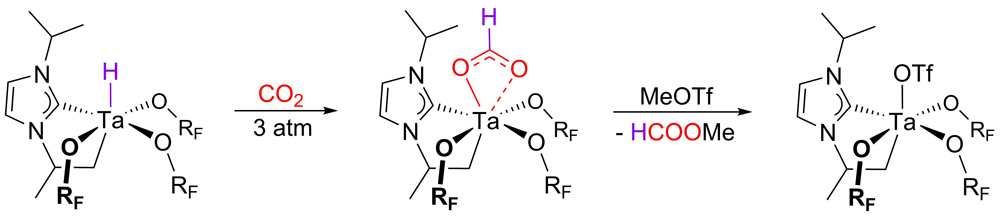
For the activation of CO2, a tailor-made tantalum hydrido complex with a cyclometallated NHC-iPr-substituent was developed. Fluorinated alkoxides (ORF = OtBu-F6) were found to be particularly suited for shielding the metal hydrido moiety. Upon reaction with carbon dioxide, this spatial shielding prevented the formation of a bridging methanediolate, which led to the isolation of the first tantalum formate starting from CO2. Remarkably, the release of methlyformate (see figure below) and the subsequent alkylation of the resulting tantalum triflate with dimethylzinc were also successful.
Homogeneous Catalysis with Early Transition Metal Complexes
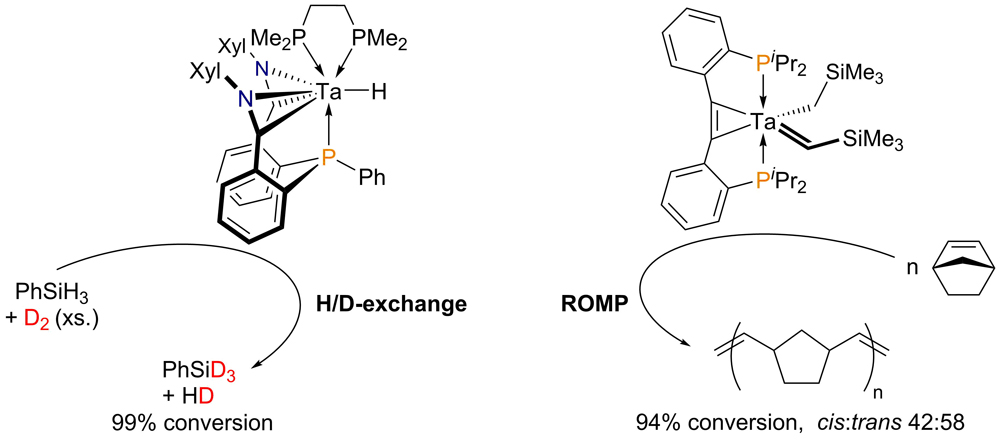
In this rather fundamental research project, we are working with reactive hydrido-, alkyl-, alkylidene- and alkylidine-complexes of the early transition metals. Special emphasis is set on the synthesis of new complexes, which are then studied with respect to their reactivity. Based on the knowledge that arrises from our reactivity studies, we start to specifically search for applications in cataylsis. To stabilize the catalysts, we use diphosphinotolanes (C/P hybrid ligands), amidophosphines (N/P hybrid ligands) and phosphino alkoxides (O/P hybrid ligands) as ligand scaffolds. Two of our tantalum catalysts are presented in the following figure.
|
|
|
Oxidized Diphosphadibenzo[a,e]pentalenes as Novel Functional Materials
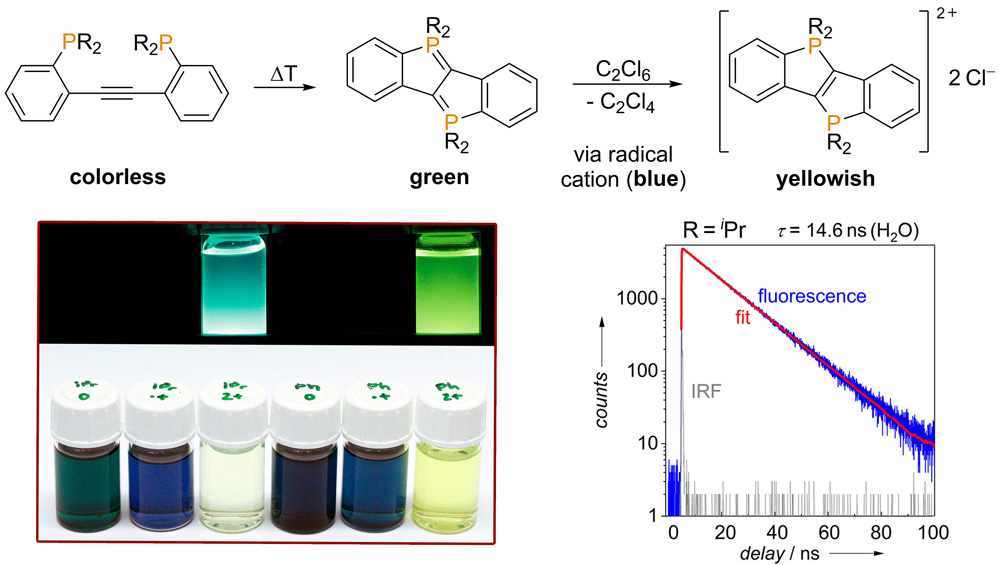
While working with diphosphinotolanes, we found that semi- or bilateral cyclizations of the ligand may occur under certain conditions. The former process may be exploited for the synthesis of new P-ylidic carbene complexes, while diphosphadibenzo[a,e]pentalenes are formed via the latter twofold cyclization (see figure below). Oxidation of these diphosphadibenzo[a,e]pentalenes led to the corresponding radical cations (1e--oxidation) and to the corresponding P-bridged ladder stilbenes (2e--oxidation), respectively. The doubly oxidized P-bridged ladder stilbenes are particularly soluble in water and exhibit a greenish-blue fluorescence, which allows for their use as new functional materials.